To remove the drawbacks of Rutherford’s atomic model and to explain structure of atom in detail Neils Bohr in 1912 proposed a model of atom. The special features of Bohr’s model are given below:
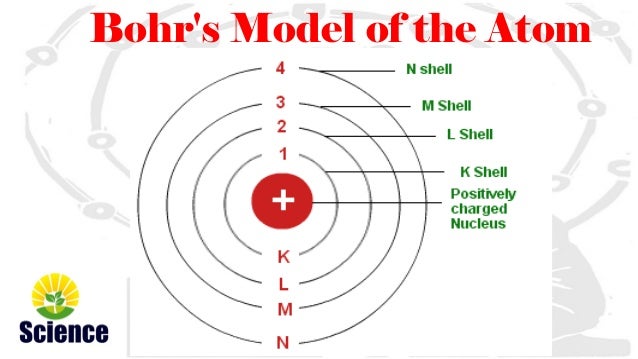
i) An electron revolves in the orbit of atom with well-defined energy.
ii) Energy of orbits increases from inner shell to the outer shells i.e. energy for orbit nearest the nucleus is lowest.
iii) If energy is supplied then electron moves from lower orbit to the higher orbit and if an electron jumps from higher orbit (energy level) to the lower orbit (energy level) then energy is radiated as electromagnetic waves.
iv) Each orbit or shell represents an energy level. Such orbits are represented as K,L,M,N,O……….. and named from centre to outwards.
v) The shell or orbits are associated with certain amount of energy and energy of orbits/shells increases from inward to outwards.eg K<L<M<N<O…………


Post a Comment
0 Comments